Is Nitrogen Combustible? Unpacking The Science Behind This Common Misconception
Let’s dive right into the big question: Is nitrogen combustible? If you’ve ever wondered whether this element can go up in flames or if it’s as harmless as it seems, you’re not alone. Nitrogen is one of the most abundant elements on Earth, making up about 78% of the air we breathe. But does that mean it’s a fire starter? Spoiler alert: It’s not. In fact, nitrogen is quite the opposite. It’s actually known for its non-combustible nature, which makes it a pretty cool player in the world of chemistry.
Now, before we get too deep into the science, let’s break it down in a way that even your non-science-y friends can understand. Nitrogen is a gas that loves hanging out in its diatomic form (N₂), which means it’s super stable. This stability makes it a tough cookie when it comes to reacting with other elements, especially when it comes to burning. So, if you’re picturing nitrogen as some kind of explosive gas, think again. We’re here to set the record straight.
But why does this matter? Well, understanding whether nitrogen is combustible isn’t just a fun trivia question. It has real-world implications, especially in industries like aerospace, manufacturing, and even your everyday life. So, buckle up because we’re about to take you on a journey through the world of nitrogen, its properties, and why it’s not going to burst into flames anytime soon.
- Nick Kyrgios Family Faith Unveiling Norlailas Religion
- Max 2024 Sudeeps Action Thriller Story Cast Movierulz Info
Understanding Nitrogen: The Basics
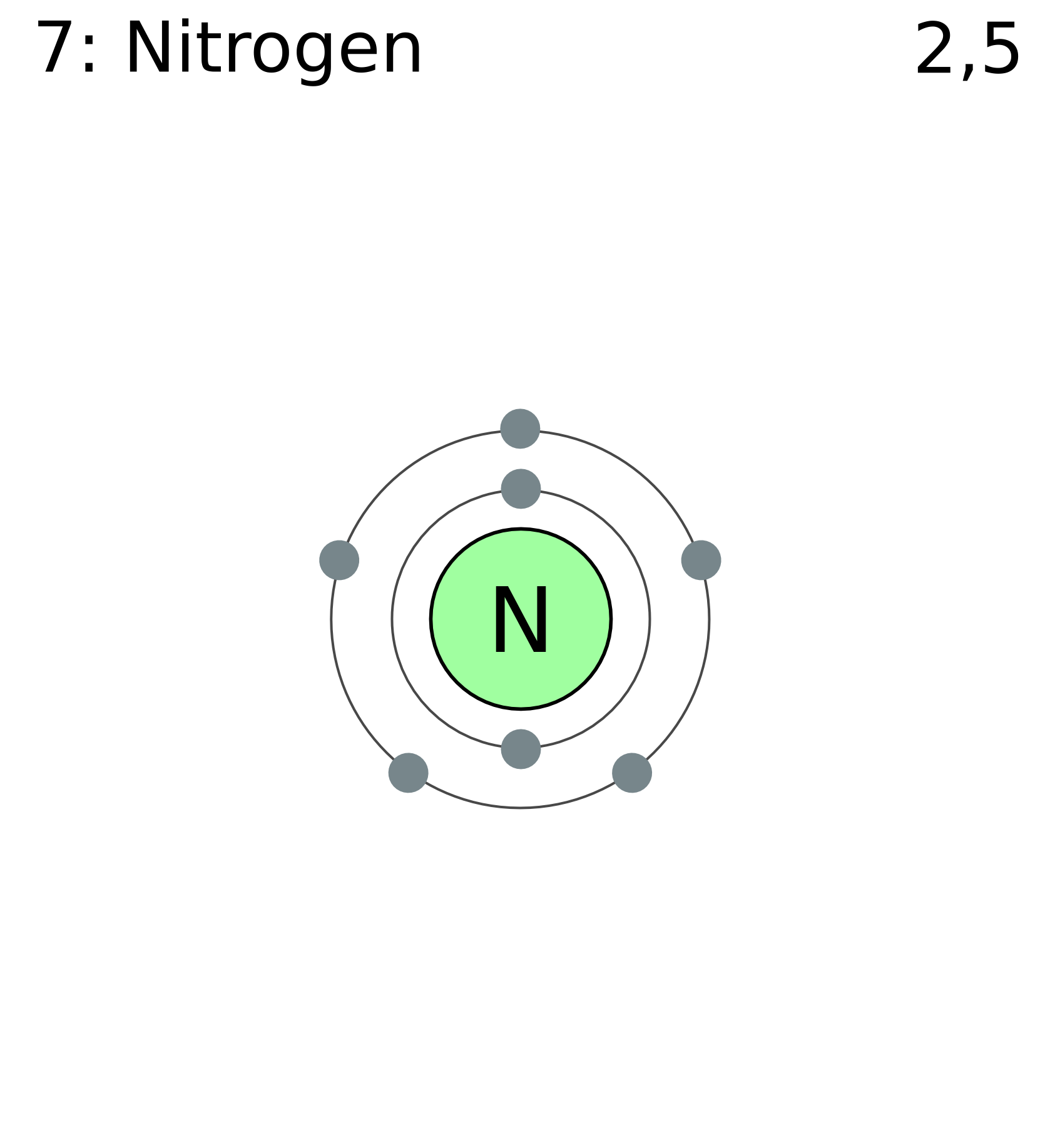
Before we dive into whether nitrogen is combustible, let’s first get to know this element a little better. Nitrogen is a colorless, odorless, and tasteless gas that makes up the majority of our atmosphere. It’s represented by the symbol "N" on the periodic table and has an atomic number of 7. But what makes nitrogen so special? For starters, it’s incredibly stable, which is why it’s often used in situations where you want to avoid combustion or oxidation.
What Makes Nitrogen So Stable?
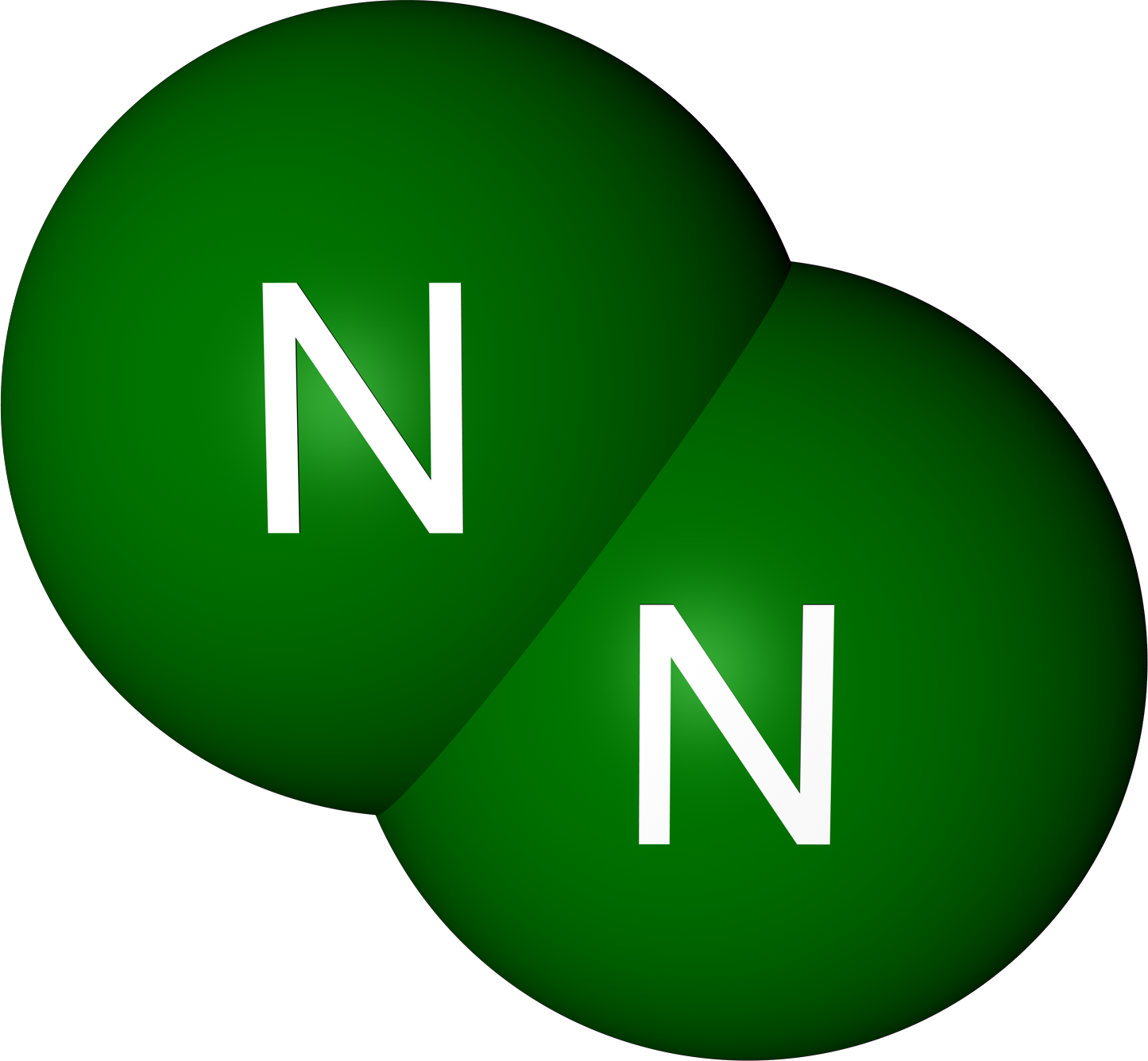
The stability of nitrogen comes down to its molecular structure. Nitrogen exists naturally as N₂, where two nitrogen atoms are held together by a triple bond. This triple bond is one of the strongest in chemistry, making it extremely difficult for nitrogen to react with other elements. Think of it like a super-strong friendship that’s hard to break. This stability is why nitrogen is considered inert in most situations.
But here’s the kicker: while nitrogen itself isn’t combustible, it can play a supporting role in combustion reactions. For example, during a fire, nitrogen can combine with oxygen to form nitrogen oxides (NOₓ), which are harmful pollutants. So, while nitrogen isn’t the main culprit in combustion, it can still have an impact on the environment.
- Movierulz Illegal Movie Sites Avoid These Risks Alternatives
- Movies More Whats Hot What To Watch Where To Stream
Is Nitrogen Combustible? Debunking the Myth
Now, let’s get to the heart of the matter. Is nitrogen combustible? The short answer is no. Nitrogen is not combustible. In fact, it’s often used as a fire suppressant in various applications. But why is that? To understand this, we need to look at what combustion actually is.
What Is Combustion?
Combustion, or burning, is a chemical reaction that occurs when a fuel combines with oxygen to produce heat and light. For combustion to happen, you need three things: fuel, oxygen, and an ignition source. This is often referred to as the fire triangle. Nitrogen doesn’t fit into this equation because it doesn’t act as a fuel or an oxidizer. Instead, it’s a bystander in the combustion process.
In fact, nitrogen is often used to displace oxygen in environments where combustion needs to be prevented. For example, in food packaging, nitrogen is used to remove oxygen and prevent spoilage. It’s also used in fire extinguishing systems to smother flames by reducing the amount of oxygen available.
Applications of Nitrogen in Everyday Life
So, if nitrogen isn’t combustible, what is it used for? The answer might surprise you. Nitrogen has a wide range of applications in everyday life, from food preservation to medical treatments. Here are a few examples:
- Food Packaging: Nitrogen is used to replace oxygen in food packaging to prevent spoilage and extend shelf life.
- Tire Inflation: Nitrogen is often used to inflate tires in aircraft and high-performance vehicles because it’s less likely to leak and maintain pressure over time.
- Medical Uses: Liquid nitrogen is used in cryotherapy to freeze and remove abnormal tissue, such as warts or skin lesions.
- Electronics Manufacturing: Nitrogen is used in the production of semiconductors and other electronic components to prevent oxidation.
As you can see, nitrogen plays a crucial role in many industries, and its non-combustible nature makes it an ideal choice for applications where safety is a top priority.
Common Misconceptions About Nitrogen
Despite its widespread use, there are still a lot of misconceptions about nitrogen. One of the most common is the belief that nitrogen is combustible. This misconception likely stems from the fact that nitrogen is involved in some combustion reactions, such as the formation of nitrogen oxides. However, as we’ve already discussed, nitrogen itself doesn’t burn.
Why Do People Think Nitrogen Is Combustible?
One reason people might think nitrogen is combustible is because of its role in nitrogen oxides (NOₓ). When fuels burn in the presence of nitrogen and oxygen, nitrogen oxides can form as a byproduct. These compounds are harmful to the environment and contribute to air pollution. However, this doesn’t mean that nitrogen itself is combustible. It’s simply a bystander in the reaction.
Another reason for the confusion might be the use of nitrogen in certain industrial applications. For example, liquid nitrogen is sometimes used in cooling systems, which might give the impression that it’s involved in combustion. In reality, liquid nitrogen is used to absorb heat, not produce it.
The Science Behind Nitrogen’s Non-Combustibility
Now that we’ve debunked the myth, let’s take a closer look at the science behind nitrogen’s non-combustibility. As we mentioned earlier, nitrogen exists naturally as N₂, where two nitrogen atoms are held together by a triple bond. This bond is incredibly strong, making it difficult for nitrogen to react with other elements.
Why Doesn’t Nitrogen React Easily?
The strength of the triple bond in N₂ means that nitrogen requires a lot of energy to break apart and form new compounds. In most cases, this energy isn’t available, which is why nitrogen remains inert in many situations. Even in high-temperature environments, such as during combustion, nitrogen is unlikely to react unless specific conditions are met.
For example, in internal combustion engines, nitrogen can combine with oxygen to form nitrogen oxides. However, this only happens at extremely high temperatures and pressures. Under normal conditions, nitrogen remains stable and non-reactive.
Environmental Impacts of Nitrogen
While nitrogen itself isn’t combustible, its role in the formation of nitrogen oxides can have significant environmental impacts. Nitrogen oxides are a major contributor to air pollution, smog, and acid rain. They can also contribute to the depletion of the ozone layer and global warming.
How Can We Reduce Nitrogen Oxide Emissions?
Reducing nitrogen oxide emissions is a key challenge for industries and governments around the world. Here are a few strategies that are being used:
- Catalytic Converters: These devices are used in vehicles to convert nitrogen oxides into harmless nitrogen and oxygen.
- Low-Emission Technologies: Industries are adopting technologies that reduce nitrogen oxide emissions, such as selective catalytic reduction (SCR) systems.
- Regulations: Governments are implementing stricter regulations on emissions to encourage the adoption of cleaner technologies.
By addressing the issue of nitrogen oxide emissions, we can help protect the environment and improve air quality for future generations.
Conclusion: Why Understanding Nitrogen Matters
In conclusion, nitrogen is not combustible. Its stability and non-reactive nature make it an ideal choice for applications where safety is a priority. However, its role in the formation of nitrogen oxides highlights the importance of understanding its environmental impacts.
So, the next time someone asks you, "Is nitrogen combustible?" you can confidently say no. And if you want to take things a step further, share this article with your friends and family to help spread the word. Who knows? You might just spark a conversation about the fascinating world of chemistry.
And don’t forget to check out our other articles for more insights into the world of science and technology. Your curiosity is the key to unlocking new knowledge, so keep exploring!
Table of Contents
- Understanding Nitrogen: The Basics
- What Makes Nitrogen So Stable?
- Is Nitrogen Combustible? Debunking the Myth
- What Is Combustion?
- Applications of Nitrogen in Everyday Life
- Common Misconceptions About Nitrogen
- The Science Behind Nitrogen’s Non-Combustibility
- Environmental Impacts of Nitrogen
- How Can We Reduce Nitrogen Oxide Emissions?
- Conclusion: Why Understanding Nitrogen Matters
- Movierulz Alternatives Watch Movies Online Free Hd
- Kannada Movies Online Finding The Best Streams Movierulz Alternatives
Nitrogen Generators How It Works CGT
Collection of Nitrogen PNG. PlusPNG
Collection of Nitrogen PNG. PlusPNG